Research
Research within the group is in 4 main areas and is supported by the EPSRC, CisBio and The Royal Society.
i) Lanthanide Complexes as Chiral Probes and Labels
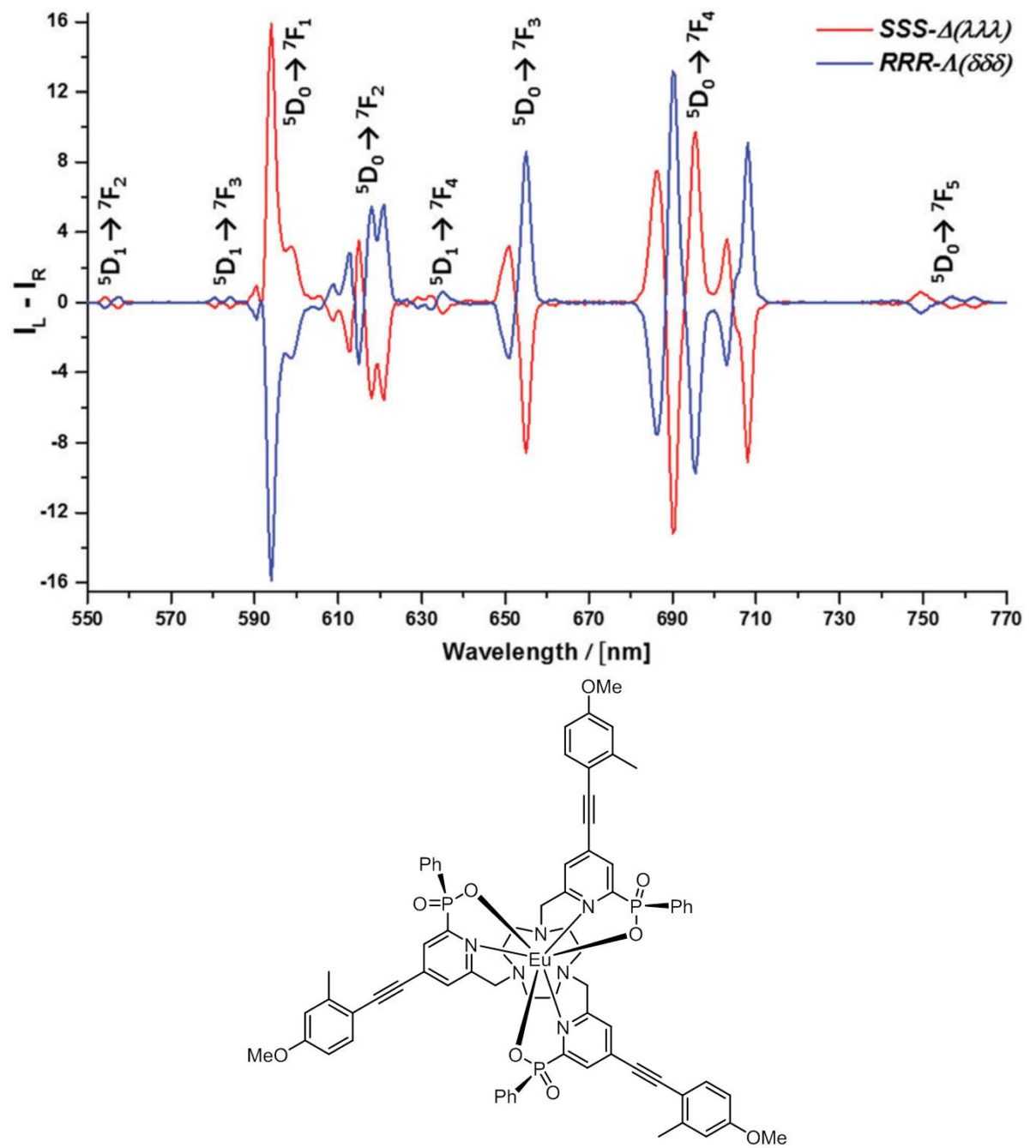
Since Faraday first declared that ‘polarised light was a most delicate investigator of molecular condition’, scientists have sought to exploit the intrinsic handedness of light. Light that is polarised in a plane is the sum of left and right handed circularly polarised components. The exploitation of the circular polarisation of light is rare, yet it offers unique opportunities in areas such as the development of optical probes for the biosciences, in the creation of new security labelling/tagging features and in the development of image contrast, based on the relative intestines of the left and right handed components of polarised light. Circularly polarised luminescence (CPL) is the emission analogue of circular dichroism (CD), which has been used for some time to examine chiral systems by virtue of the differential absorption of left and right handed light. Intrinsically, CPL is a much more sensitive technique, but has not really been used at all in life or material science applications.
In Durham, very bright compounds of the rare earth element europium have recently been created that are not only the brightest emitters of red light that have been devised, but also emit light with a preferential handedness. Using these new compounds, it is much faster and easier to detect their CPL, and new instrumentation has been developed in tandem allowing their CPL behaviour to be studied both in spectroscopy and in microscopy. Methods of using these bright red-emitting emitters of light to tag or label documents or printed labels are being examined, thereby opening up the possibility of their use in security applications for validation of true identity. Examples might include bank notes, high-end branded labels for designer clothing or official documents, such as passports.
In addition, new molecules will be designed and created that bind to a wide range of enzymes in the body that are involved in the transfer of a phosphate group. For example, the protein tyrosine phosphatases account for 0.05% of the total phosphorylation in cells, but play a key role in the regulation of critical biological functions, e.g. adhesion, cell cycle control and the ways that cells grow and differentiate. Antibodies specific to phosphorylated tyrosine sites are commonly used for many practical applications, involving monitoring enzyme activity, but their use is hampered by high cost and poor stability. Chemical probes that can directly visualise the activity of this specific range of enzymes activity are required and could be used in drug screening applications, as the inhibitors of these key enzymes are of great interest to the pharmaceutical sector. Using europium compounds that interact selectively with these sites where tyrosine has been phosphorylated, a chiral CPL signature can be observed, that identifies the site and the nature of the amino-acids around that site. Thus the europium compounds can serve as selective chemical probes to signal, by an induced CPL response, whether the tyrosine is phosphorylated and where in the biomolecule it has been modified. These properties are being studied in detail and their scope and utility evaluated.
ii) EuroTracker Dyes
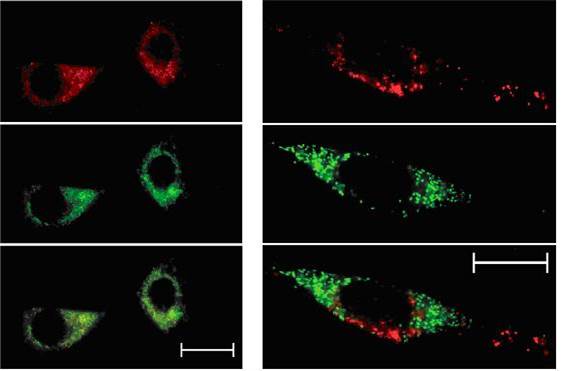
The visualisation of certain living cells or microbes by optical microscopy requires the introduction of bright, luminescent dyes or stains, that can light up selected parts of the cell effectively. We are developing bright complexes of europium (EuroTracker dyes) as optical probes that emit light efficiently and report information to the observer about their chemical or biological environment. These probes have been designed to allow them to be excited by light sources that are available in microscopes used by physical scientists, biochemists and cell biologists and to study the interior composition of cells or related small compartments. By examining series of closely related probes, we shall define systems that locate to a particular compartment of a cell, allowing it to be visualised selectively.
The optical probes that are currently used are based either on organic compounds, on large man-made proteins or on so-called quantum dots; each of these probes suffer from problems of low chemical stability, a tendency to fade and, in certain cases, they are toxic to living cells. Thus, we aim to change the thinking of the microscopy community and to pave the way for such emissive metal complexes to be used as stains and probes in biology. These metal complex probes emit light over longer timescales than conventional probes, and so there is more time to collect the emitted light after pulsed excitation. The long emission lifetime avoids problems in data acquisition that are associated with light scattering and background auto-fluorescence.
In parallel, Dr Robert Pal (also in Durham) is developing instrumentation methods that allow the spectral signature of these probes to be recorded quickly using fast imaging methods. This will allow changes in chemical composition within a cell to be tracked on a 10 second timescale. As a first step, we are developing probes that can report on pH change and on the amount of sodium, magnesium and zinc within a specific cell compartment, allowing changes in the amount of these important metal ions to be tracked in living cells in real time. By adopting a modular approach to probe design, different metal ions can be examined, by introducing the appropriate metal-binding moiety into the probe structure.
iii) Non-classical paramagnetic susceptibility and anisotropy in lanthanide coordination complexes: a combined experimental and theoretical study
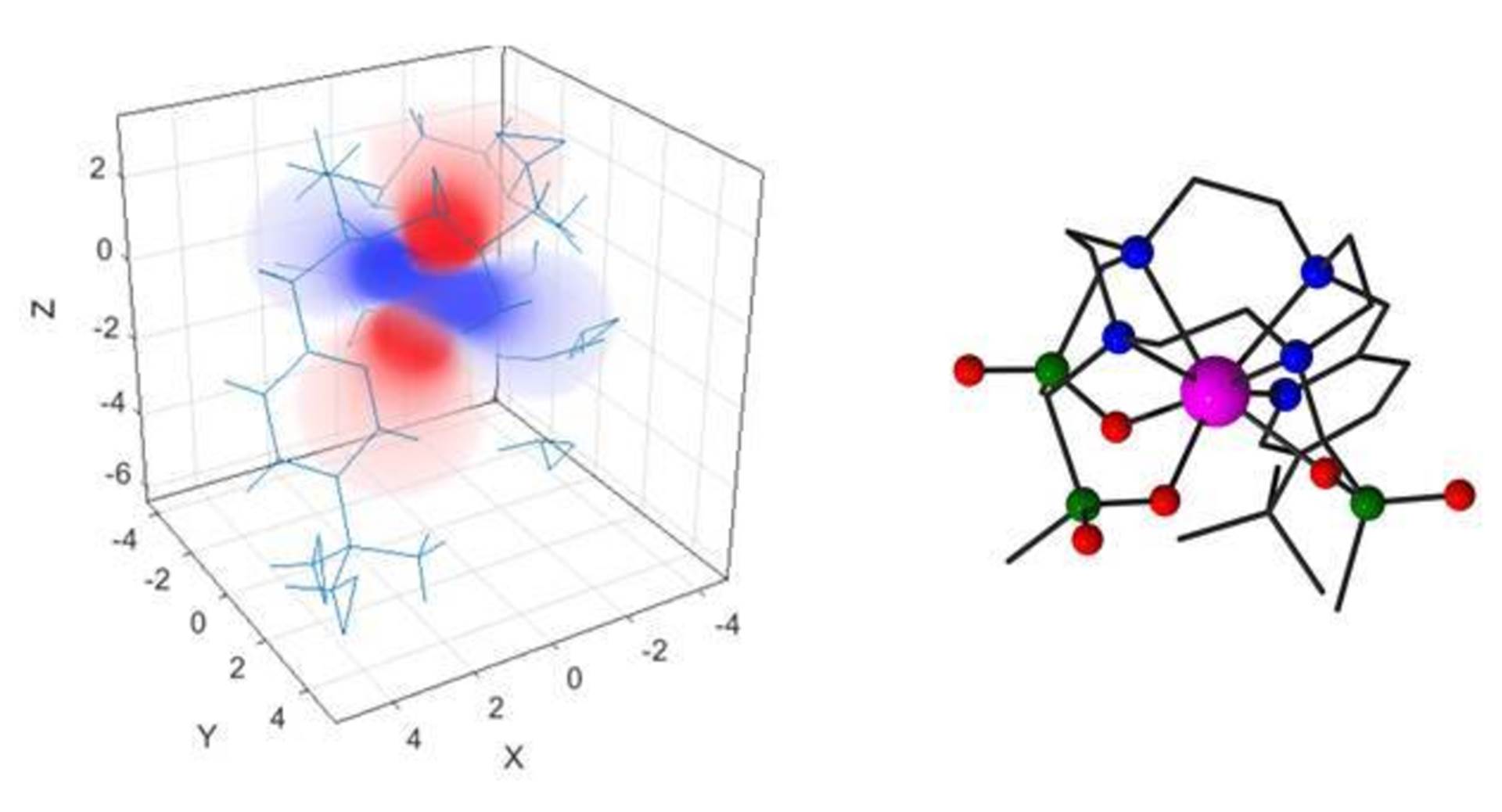
Rare earth elements are used widely in society and industry in the 21st century. Your mobile phone contains up to 9 different rare earth elements, harnessing their unique magnetic and optical properties. Advances in the application of these properties require that we understand better the physicochemical origins of their behaviour. A key part of this process is to develop new theories that test our understanding, and guide us in the design of new chemical applications.
Of particular importance is the magnetic behaviour of the rare earth elements in their chemical compounds, and the ramifications of the directional dependence of the ‘paramagnetism’ that arises from unpaired electron density. This behaviour has important consequences not only in the design of new magnetic materials, but also in their use in magnetic resonance imaging (MRI), where they have been used since 1988 as contrast agents to assist in clinical diagnosis of disease. For example, paramagnetic lanthanide coordination complexes are being created as proton chemical shift magnetic resonance probes, in a radical change in imaging technology that directly relates to the importance of imaging technologies in healthcare.
This multidisciplinary project brings together three teams of scientists with complementary expertise in Durham, Manchester and Southampton to develop and test new theoretical and computational approaches. This will promote a better understanding of the magnetic properties of new series of rare earth chemical compounds that are directly relevant to their application in magnetism and their scope for use in MRI.
iv) PARASHIFT Probes: Triple Imaging in MRI
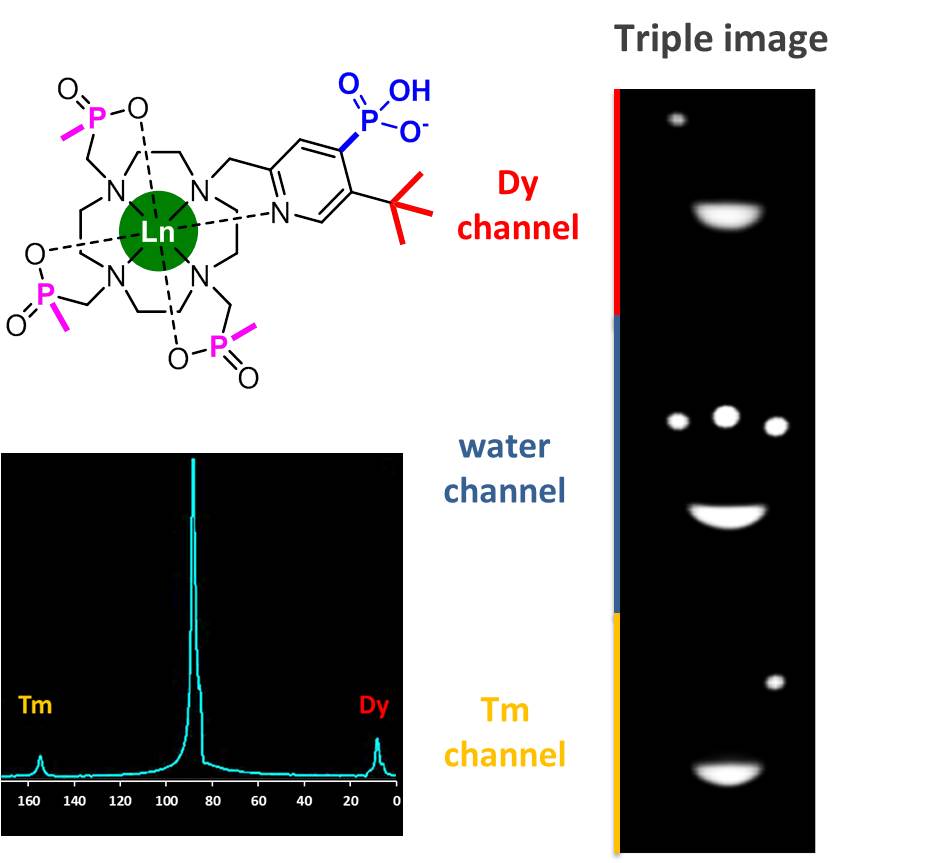
Magnetic resonance imaging (MRI) affords a window to the human body reporting the relative distribution of water in tissues within the body. MRI scans are used for clinical diagnosis with 100 million examinations per year. 40% use a contrast agent that is given to the patient to enhance image clarity, aiding the radiologist in interpreting the results. The scanner is tuned to the frequency where the water hydrogen nuclei resonate, and lies in the radiofrequency range.
We are developing, with Professor Andrew Blamire in Newcastle, a series of contrast agents that can be simultaneously observed with the water signal. These contrast agents, based on rare earth metal complexes, possess an intense reporting proton signal that can be observed far away from the water signal, typically 10 to 20 kHz away, allowing their selective observation. Careful design of their molecular structure allows the probe resonant frequency to be made sensitive not only to local temperature but also to other physiological parameters, such as pH or the local metal ion concentration. By administering two rare earth complexes at the same time, containing different metals (such as thulium/dysprosium or erbium/terbium), shifted reporter group signals can be observed simultaneously with the water signal. This is termed a triple imaging experiment. By measuring the two probe frequencies, both the temperature and the pH can be assessed in the region of interest that is studied. In general, local temperature increases in the liver or kidney are indicators of disease, especially inflammation, as occurs in hepatitis or fibrosis of the liver. Similarly, altered extracellular pH is indicative of tissue ischaemia as occurs in many diseases where blood flow has been restricted, e.g. coronary heart disease and end stage renal disease.
A key aspect of this ‘triple imaging’ approach is that the new contrast agents can be detected at relatively low concentration, and at levels that are safe to use. These levels lie within the current range of the approved gadolinium contrast agents based on a cyclic ring structure that have been used clinically since 1988. This enhanced sensitivity arises from the proximity of the reporting proton signalling group to a magnetic metal centre that is incarcerated within the contrast agent: the signal acquisition sequence can be shortened, allowing signal intensity to be acquired about 20 times faster than otherwise possible.
